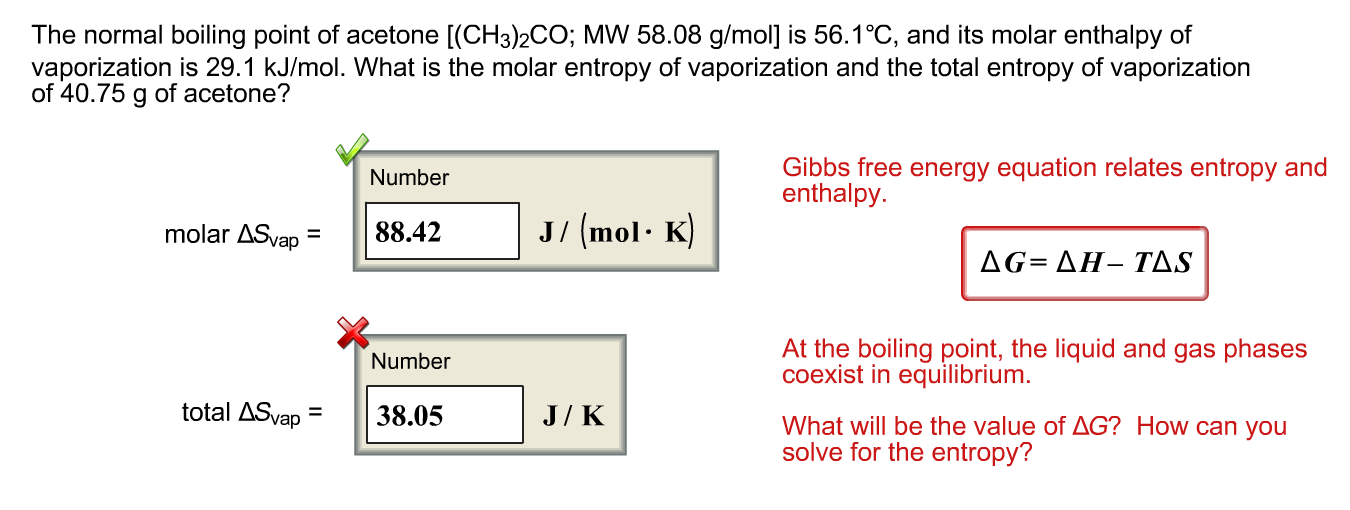
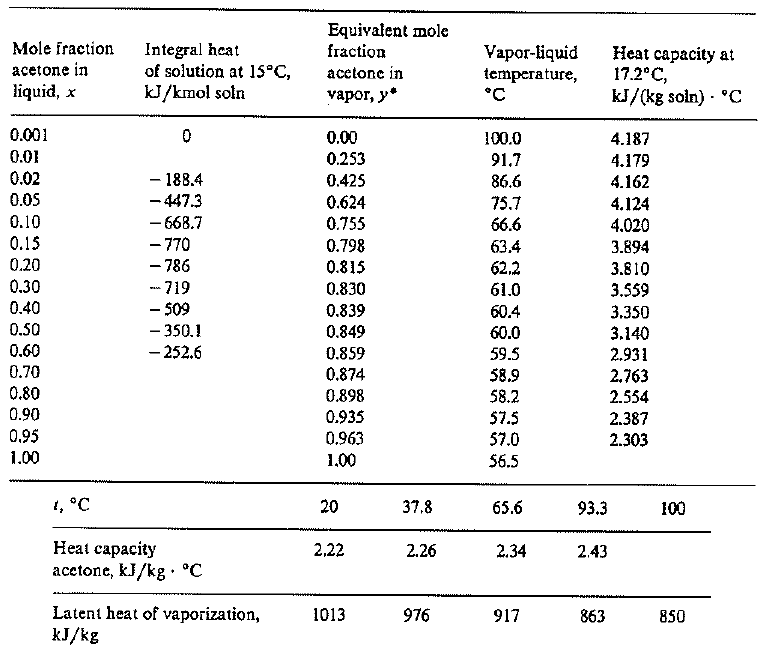
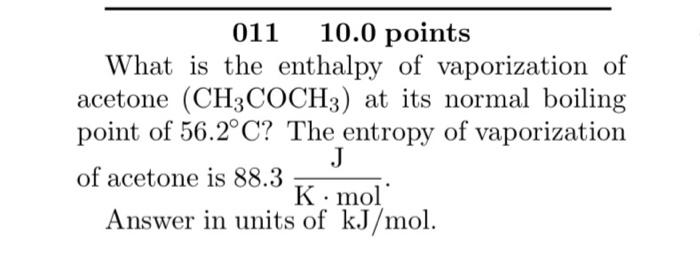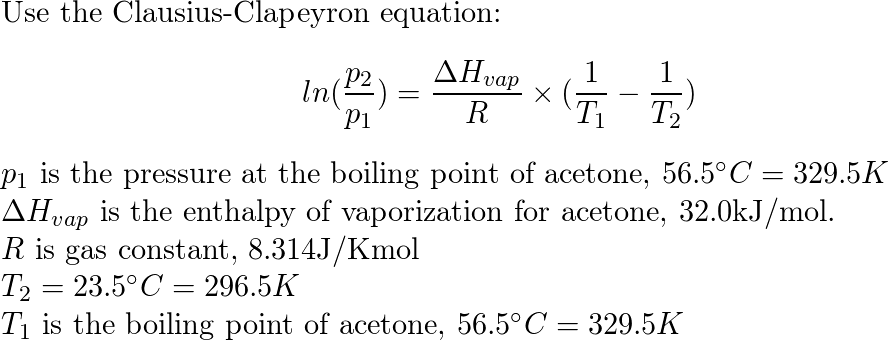SOLVED: The standard heat of vaporization of acetone is 31.3 kJ/mol and its normal boiling point is 56°C. Calculate the standard entropy of vaporization. 10.5 J/mol K 0.095 J/mol K 559 J/mol

Melting Point, Boiling Point, and Heat of Vaporization of Some Common... | Download Scientific Diagram

SOLVED:The molar heat of vaporization of acetone, C3 H6 O, is 30.3 kJ / mol at its boiling point. How many kilojoules of heat would be liberated by the condensation of 5.00

The enthalpy of vaporization for acetone is 320 kJ mol-1 The normal boiling point for acetone is 5 - YouTube

Heat of vaporization of acetone. Simulation data: • this work, AUA4... | Download Scientific Diagram

SOLVED: The enthalpy of vaporization for acetone is 32.0 kJ/mol. The normal boiling point for acetone is 56.5 °C. What is the vapor pressure of acetone at 30.58 °C? (Enter your answer

SOLVED: How much heat is required to vaporize 38.5 g of acetone (C3H6O; molar weight 58.1 g) at 25 °C? The heat of vaporization for acetone at this temperature is 31.0 kJ/mol.

OneClass: A standard entropy of vaporization of acetone is approximately 85jk/mol at its boiling poin...

SOLVED: The enthalpy of vaporization for acetone is 32.0 kJ/mol. The normal boiling point for acetone is 56.5 °C. What is the vapor pressure of acetone at 47.4°C? (Enter your answer to
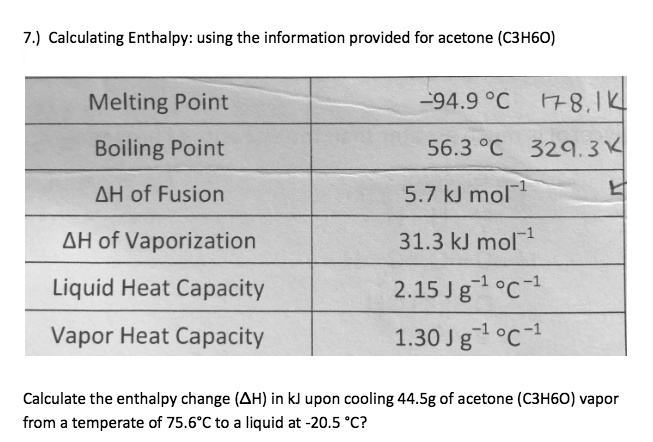
SOLVED: Calculating Enthalpy: using the information provided for acetone (C3H6O) Melting Point Boiling Point AH of Fusion 94.9 °C 56.3 °C 329.34 kJ mol-1 AH of Vaporization 31.3 kJ mol-1 2.15 J

SOLVED: If the enthalpy of vaporization of acetone is 32.0 kJ/mol, what mass of acetone (molar mass equals 58.08 g/mol) will be vaporized when 121 kJ of energy (as heat) are added