Reducing the experimental error in an experiment to determine the latent heat of vaporization of liquid nitrogen

Latent Heat of Vaporization – Delta Hvap – of Water calculated by corresponding states correlation in a one cell excel formula | Chem-Eng-Musings
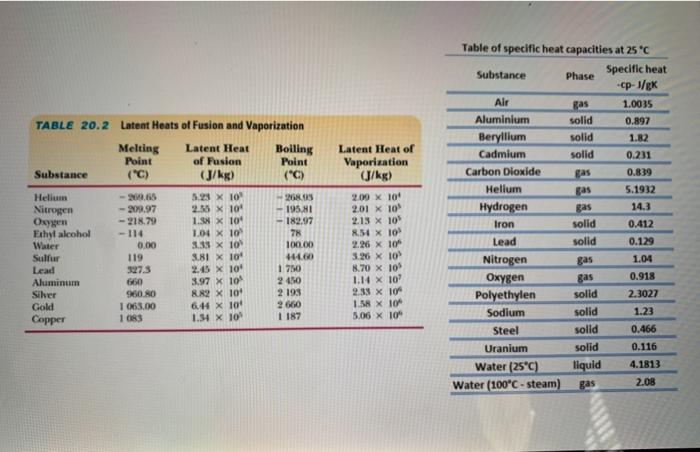
![SOLVED: Table 14.2 Heats of Fusion and Vaporization [4] Substance Melting point (°C) kJ/kg kcal/kg Boiling point (°C) kJ/kg kcal/kg Helium -269.5 5.23 1.25 -268.9 20.9 Hydrogen -259.3 58.6 14.0 -252.9 452 SOLVED: Table 14.2 Heats of Fusion and Vaporization [4] Substance Melting point (°C) kJ/kg kcal/kg Boiling point (°C) kJ/kg kcal/kg Helium -269.5 5.23 1.25 -268.9 20.9 Hydrogen -259.3 58.6 14.0 -252.9 452](https://cdn.numerade.com/ask_images/7eefeb940d43454585a290de4b60178c.jpg)
SOLVED: Table 14.2 Heats of Fusion and Vaporization [4] Substance Melting point (°C) kJ/kg kcal/kg Boiling point (°C) kJ/kg kcal/kg Helium -269.5 5.23 1.25 -268.9 20.9 Hydrogen -259.3 58.6 14.0 -252.9 452

Latent Heat of Vaporization – Delta Hvap – of Water calculated by corresponding states correlation in a one cell excel formula | Chem-Eng-Musings


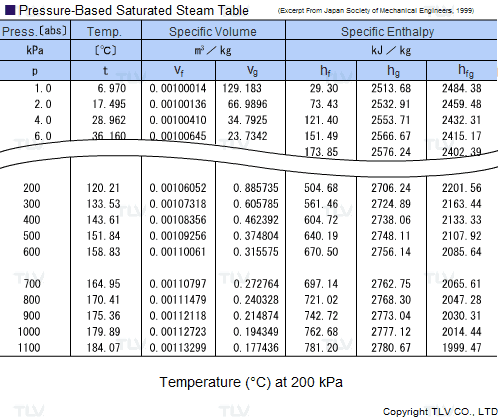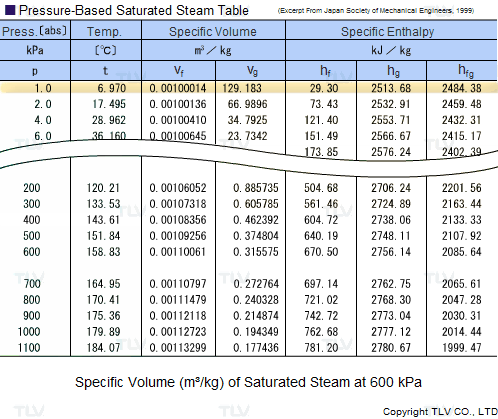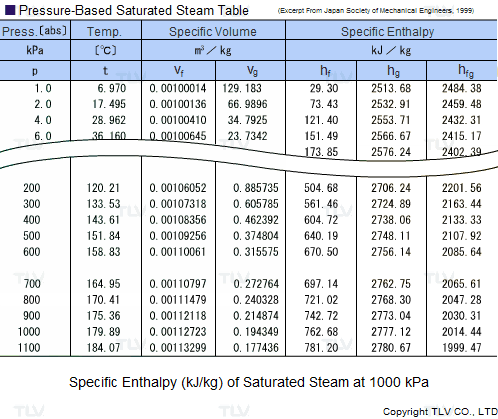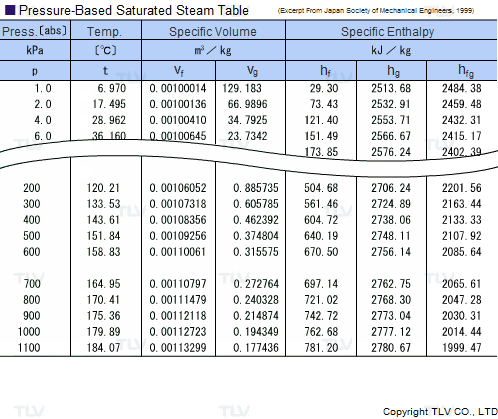

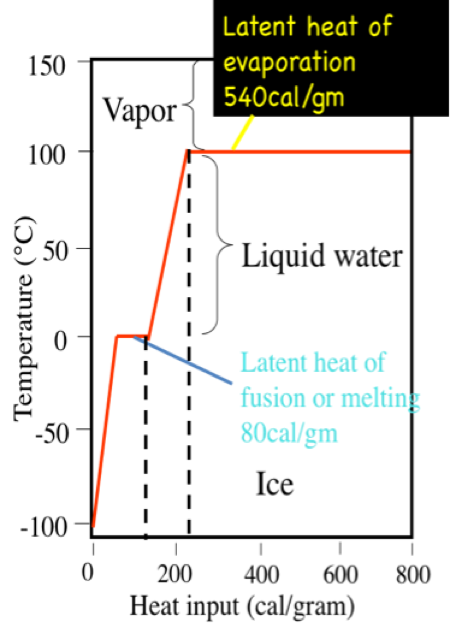
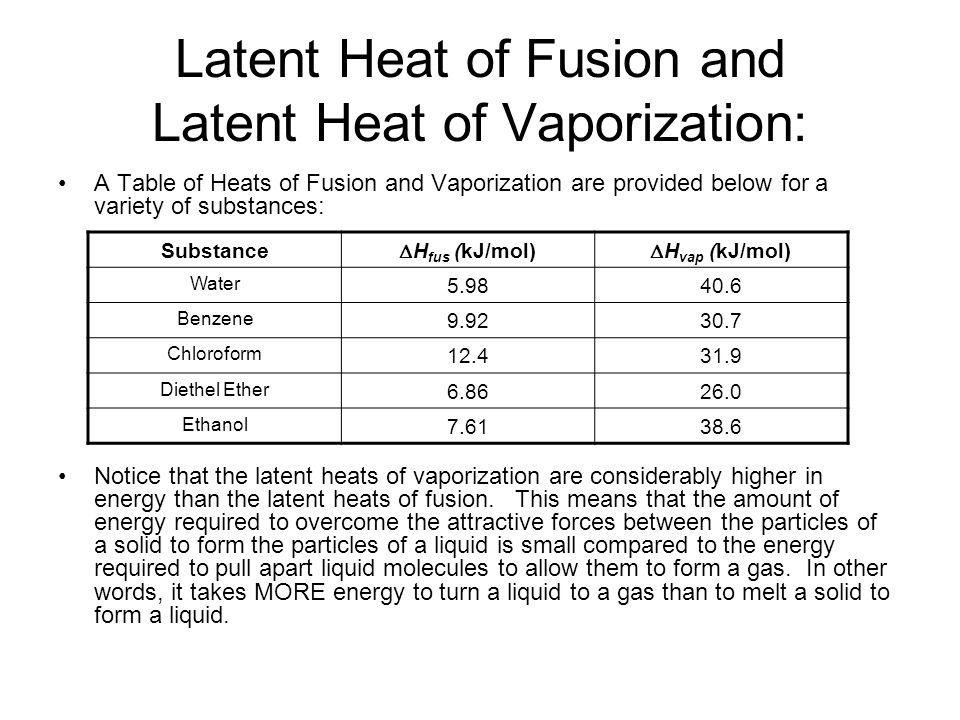
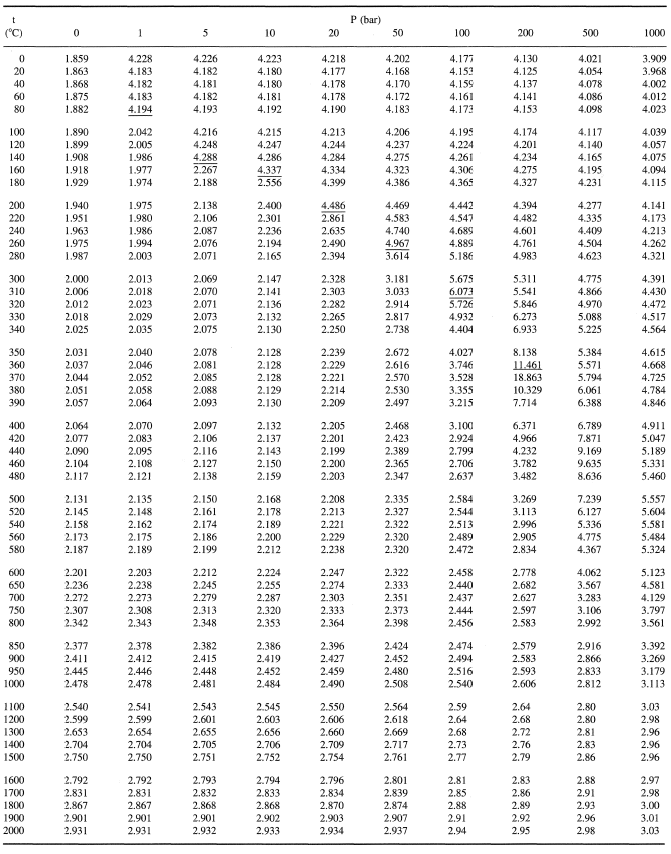
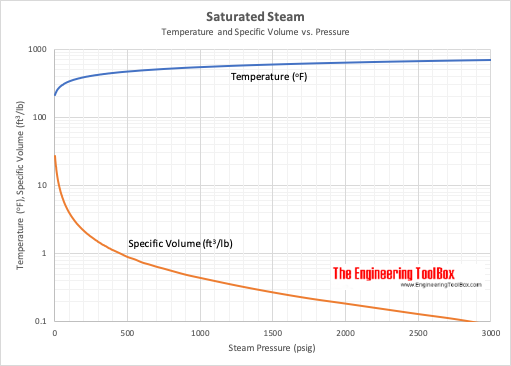

![Latent heat of vaporization for main components of LNG [10]. | Download Table Latent heat of vaporization for main components of LNG [10]. | Download Table](https://www.researchgate.net/publication/330572654/figure/tbl3/AS:718422421803010@1548296661881/Latent-heat-of-vaporization-for-main-components-of-LNG-10.png)

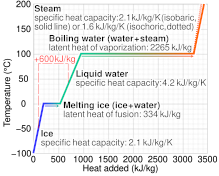

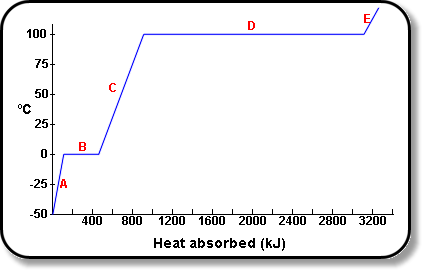

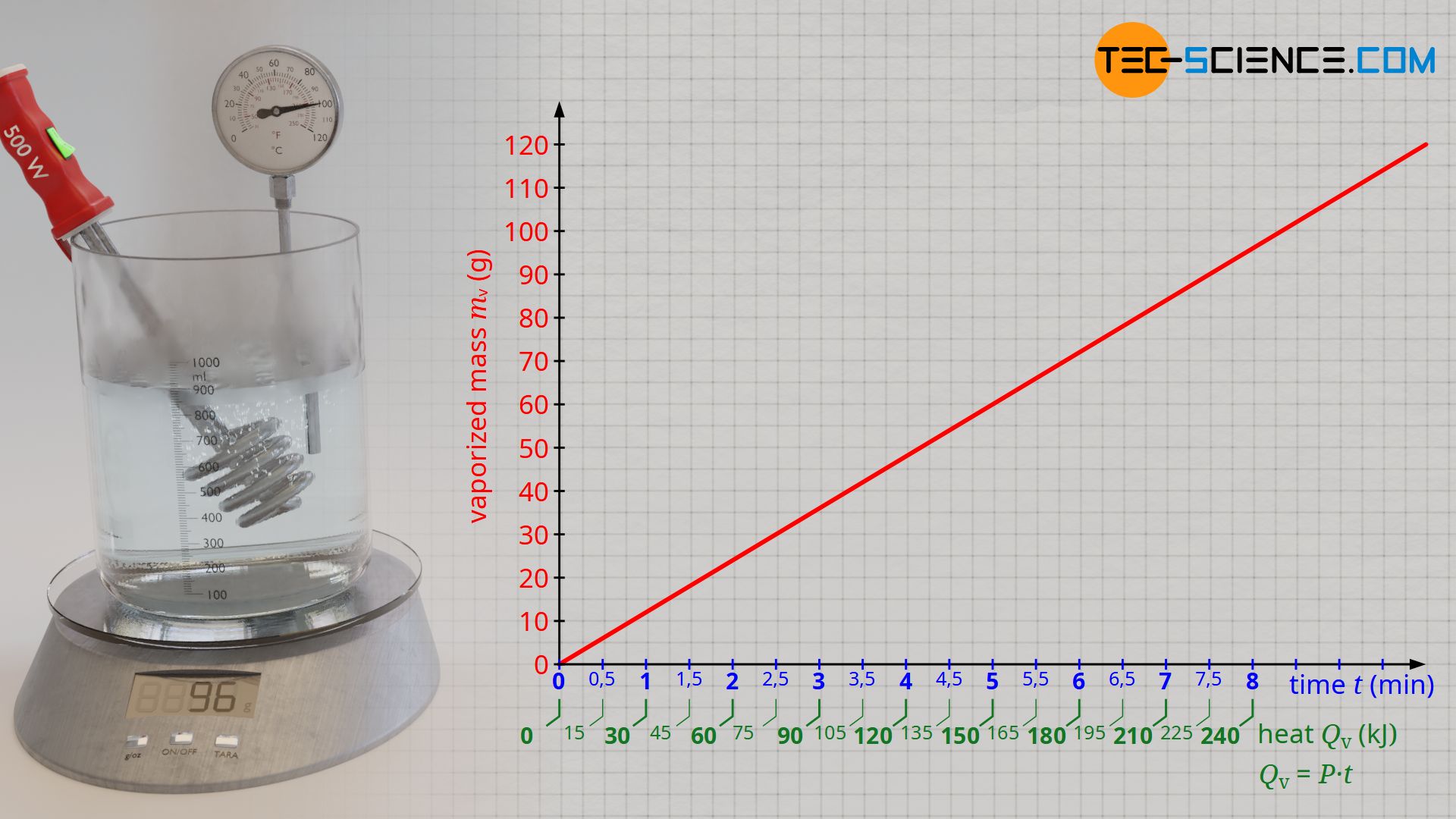


![PDF] Latent heat of vaporization for selected foods and crops | Semantic Scholar PDF] Latent heat of vaporization for selected foods and crops | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/e5e36a21914ce3ba4f27cd724afeb4e94d98ddce/3-TableI-1.png)
![Latent heat of sorption, H [kWh], released during one-day time interval... | Download Table Latent heat of sorption, H [kWh], released during one-day time interval... | Download Table](https://www.researchgate.net/publication/316049533/figure/tbl2/AS:669210078359562@1536563524629/Latent-heat-of-sorption-H-kWh-released-during-one-day-time-interval-during-water.png)